| Version 23 (modified by , 10 years ago) ( diff ) |
|---|
Quality Control and preprocessing of short reads
Analyzing short read quality (before mapping)
Quality scoring introduction
- Quality scores are typically represented using a Phred scoring scheme, where a read quality value = −10 * log10 (error probability)
- For example,
- Quality = 10 => error rate = 10% => base call has 90% confidence
- Quality = 20 => error rate = 1% => base call has 99% confidence
- Quality = 30 => error rate = 0.1% => base call has 99.9% confidence
- See Phred quality score for more details.
FastQC
http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc
- quality control analysis with nice graphical output
- available for Linux, Windows, and MacOSX
- (but no tools for editing reads)
It's installed on tak and LSF and can be run from the command line
- Sample command 1 (fastq inputs): fastqc s_1_sequence.txt s_2_sequence.txt
- Sample command 2 (fastq.gz inputs): fastqc s_1_sequence.txt.gz s_2_sequence.txt.gz
or interactively (with X Windows):
- fastqc
Output is a directory (named "s_1_sequence_fastqc" for input "s_1_sequence.txt") with "fastqc_report.html", a web page including all figures.
The "Basic Statistics" section at the top of the FastQC report also shows the Encoding (quality score) information (like "Illumina 1.5"), which may be necessary to specify in subsequent analysis steps. The Encoding scales are described at http://en.wikipedia.org/wiki/FASTQ_format#Encoding.
QC for paired-end reads
- QC as above each of the forward and reverse reads separately using QC'ing program (above).
- If reads reads are removed, get reads/mates after QC'ing that are perfect pairs:
bsub “/nfs/BaRC_Public/BaRC_code/Perl/cmpfastq/cmpfastq.pl s_8_1_sequence.txt s_8_2_sequence.txt” # fastq inputs
ShortRead (R package)
http://www.bioconductor.org/packages/release/bioc/html/ShortRead.html
R package, Linux (Tak), Window, Mac
- It takes the fastq files, and creates a website with different ways to check the data, and with instruction on how to interpret results. The output files are stored in dest folder (my_qa in this example).
- QC a single file using ShortRead
library("ShortRead")
# load the data
sr <- readFastq("s1_sequence.txt")
# create a qa object from the ShortRead object
qa <- qa( sr, lane="character" )
# create an html report in the qa directory
report(qa, dest="my_qa")
- QC all *.txt fastq files in a directory using ShortRead.
library("ShortRead")
qaSummary <- qa(".", pattern="*.txt", type="fastq")
#create an html report in the qa directory
report(qaSummary, dest="myQC_dir")
Fastx Toolkit
http://hannonlab.cshl.edu/fastx_toolkit/
galaxy integration, Linux(Tak), MacOSX
# Sample commands: # quality_stats: Sample Solexa reads file: s_1_1_sequence.txt or s_1_1_sequence.txt.gz fastx_quality_stats -i s_1_1_sequence.txt -o s_1_1_sequence.stats # fastq input gunzip -c s_1_1_sequence.txt.gz | fastx_quality_stats -o s_1_1_sequence.stats # fastq.gz input # a figure for Nucleotide Distribution: fastx_nucleotide_distribution_graph.sh -i s_1_1_sequence.stats -o s_1_1_sequence.stats.nuc.png -t "s_1_1_sequence.stats Nucleotide Distribution" # boxplot: fastq_quality_boxplot_graph.sh -i s_1_1_sequence.stats -o s_1_1_sequence.stats.quality.png -t "s_1_1_sequence.stats Quality Scores"
Modifying a file of short reads based on quality considerations
Remove reads with low quality score: To use FASTX Toolkit to get only reads that are above a quality score along with a certain read length:
fastq_quality_filter -v -q 20 -p 75 -i myFile.fastq -o myFile.fastq.fastx_trim # fastq input and output gunzip -c myFile.fastq | fastq_quality_filter -v -q 20 -p 75 -z -o myFile.fastq.fastx_trim.gz # fastq.gz input and output [-h] = This helpful help screen. [-q N] = Minimum quality score to keep. [-p N] = Minimum percent of bases that must have [-q] quality. [-z] = Compress output with GZIP. [-i INFILE] = FASTA/Q input file. default is STDIN. [-o OUTFILE] = FASTA/Q output file. default is STDOUT. [-v] = Verbose - report number of sequences. If [-o] is specified, report will be printed to STDOUT. If [-o] is not specified (and output goes to STDOUT), report will be printed to STDERR.
If you get an error like "Invalid quality score value", your fastq file probably has Sanger (offset 33) instead of Illumina (ASCII offset 64) quality scores. You'll need to add the option "-Q33" to your FASTX Toolkit arguments.
Trim end of reads when quality drops below a threshold
- sample command:
bsub "fastq_quality_trimmer -v -t 20 -l 25 -i input.fastq -o output.fastq" # fastq input and output
bsub "gunzip -c input.fastq.gz | fastq_quality_trimmer -v -t 20 -l 25 -z -o output.fastq.gz" # fastq.gz input and output
[-t N] = Quality threshold - nucleotides with lower
quality will be trimmed (from the end of the sequence).
[-l N] = Minimum length - sequences shorter than this (after trimming)
will be discarded. Default = 0 = no minimum length.
[-z] = Compress output with GZIP.
[-i INFILE] = FASTQ input file. default is STDIN.
[-o OUTFILE] = FASTQ output file. default is STDOUT.
[-v] = Verbose - report number of sequences.
If [-o] is specified, report will be printed to STDOUT.
If [-o] is not specified (and output goes to STDOUT),
report will be printed to STDERR.
Modifying a file of short reads in other ways
Remove linker (adapter) RNA:
- What is the sequence of the linker (adapter) to be removed?
- Biologists generally know which linker (adapter) RNA is used for their sample(s).
- Also or in addition, when you run quality control with shortRead or FASTQC, check out
- repetitive segments in the "over represented sequences" section.
- "Per base sequence content" for any patterns at the beginning of your reads
- cutadapt is a good tool that is designed to find and remove adapters:
- more options than fastx_clipper, such as specifically trimming 5' or 3' adapters and specifying error rate (allowed mismatches)
- much more conservative than fastx_clipper.
- sample usage
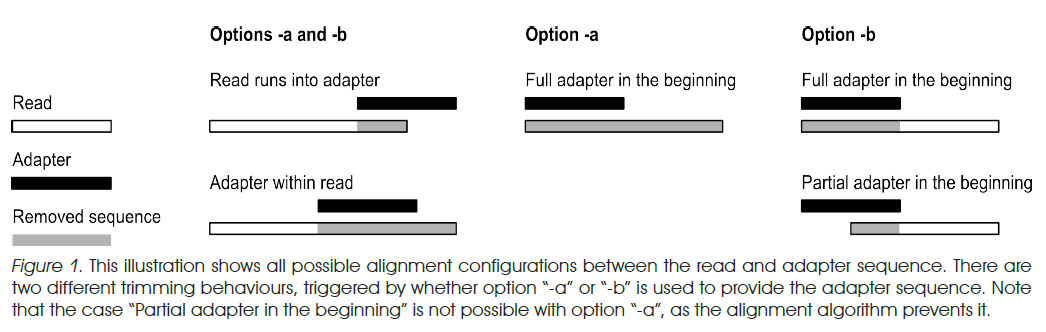
- a vs b options from EMBnet.journal
- sample command:
bsub cutadapt -a GATCGGAAGAGCTCGTATGCCGTCTT –o Nanog_noAdapter.fastq Nanog.fastq In the above command: -a: Sequence of an adapter that was ligated to the 3' end. -o: output file name
- See fastx_clipper usage (or fastx_clipper -h) for more arguments
- sample command:
bsub "fastx_clipper -a CTGTAGGCACCATCAAT -i s2_sequence.txt -v -l 22 -o s2_sequence_noLinker.txt" # fastq input and output bsub "gunzip -c s2_sequence.txt | fastx_clipper -a CTGTAGGCACCATCAAT -v -l 22 -z -o s2_sequence_noLinker.txt.gz" # fastq.gz input and output In the above command: -a CTGTAGGCACCATCAAT is the linker sequence -i s2_sequence.txt is input solexa fastq file -v is Verbose [report number of sequences in output and discarded] -l 22 is to discard sequences shorter than 22 nucleotides -o s2_ sequence_noLinker.txt is output file.
- If you get the message "Invalid quality score value..." you have the older range of quality scores.
- Add the argument -Q 33, such as
- fastx_clipper -a CTGTAGGCACCATCAAT -Q 33 -i s2_sequence.txt -v -l 22 -o s2_sequence_noLinker.txt
Trim reads to a specified length
- If we have reads of different lengths (i.e. because we clipped out the adapter sequences), we can trim them to have them all be the same length. Use fastx_trimmer for that.
- sample command:
bsub "fastx_trimmer -f 1 -l 22 -i s7_sequence_clipped.txt -o s7_sequence_clipped_trimmed.txt" # fastq input and output
bsub "gunzip -c s7_sequence_clipped.txt | fastx_trimmer -f 1 -l 22 -z -o s7_sequence_clipped_trimmed.txt.gz" # fastq.gz input and output
[-i INFILE] = FASTA/Q input file. default is STDIN.
[-o OUTFILE] = FASTA/Q output file. default is STDOUT.
[-l N] = Last base to keep
[-f N] = First base to keep. Default is 1 (=first base).
Select reads that are paired [for paired-end sequencing]
During quality control, if low-quality reads have been removed for any reason, some reads may not have a paired end at the other end. This can cause problems with mapping programs.
Sample command:
/nfs/BaRC_Public/BaRC_code/Perl/cmpfastq/cmpfastq.pl sequence.1_1.filt.txt sequence.1_2.filt.txt # fastq inputs
Output files will be
- *unique.out (reads that are only in the "1" or "2" set; 2 files) and
- *common.out (reads that are in both the "1" and "2" set; 2 files).
The *common.out reads should be used for paired-read mapping.
Galaxy
http://main.g2.bx.psu.edu/
Many functions
Analyzing potential species composition of reads in a fastq file
- To get a quick preview of what genomes/collections (or vectors or other contaminants) of sequences the reads in a fastq file can map to, one can use the FastQ Screen tool.
- Information about FastQ Screen can be found at this page: http://www.bioinformatics.babraham.ac.uk/projects/fastq_screen/
- FastQ Screen allows you to screen your fastq file against a set of libraries, which can be set up to contain the genomes of interest, potential contaminating genomes, vectors, sequencing adaptors, ribosomal RNAs, or other contaminants commonly seen in sequencing experiments. This allows you to see if composition of the reads matches with what you expect and, where the contamination might have come from, if any.
- FastQ Screen uses bowtie or bowtie2 for mapping. The libraries you wish to screen against need to be bowtie-indexed.
- Paths to the bowtie indexed libraries need to be specified in the fastq_screen.conf file, which is called either from the same directory where the fastq_screen program is (by default) or from a manually specified location using the -conf /path/to/.conf file optioin.
- Sample commands are:
"bsub fastq_screen [OPTION] file.fq" Commonly used options are: [--aligner] Specify 'bowtie' or bowtie2' to use for the mapping. [--outdir] Specify a directory in which to save output files. [--illumina1_3] Assume that the quality values are in encoded in Illumina v1.3 format. Defaults to Sanger format if this flag is not specified. [--conf] Manually specify a location for the configuration file to be used for this run. If not specified then the file will be taken from the same directory as the fastq_screen program.
Analyzing short read quality (after mapping)
Remove Duplicates
- Remove duplicates, for eg. from PCR
#samtools command samtools rmdup [-sS] <input.srt.bam> <output.bam> -s or -S depending on PE data or not
Determining the paired-end insert size for DNA samples
If paired-end insert size or distance is unknown or need to be verified, it can be extracted from a BAM/SAM file after running Bowtie.
When mapping with bowtie (or another mapper), the insert size can often be included as an input parameter (example for bowtie: -X 500), which can help with mapping. See the mapping SOP for mapping details.
Method 1: Get insert sizes from BAM file
# Using a SAM file (at Unix command prompt)
awk -F "\t" '$9 > 0 {print $9}' s_1_bowtie.sam > s_1_insert_sizes.txt
# Using a BAM file (at Unix command prompt)
samtools view s_1_bowtie.bam | awk -F"\t" '$9 > 0 {print $9}' > s_1_insert_sizes.txt
# and then process column of numbers with R (or Excel)
# In R Session
sizeFile = "s_1_insert_sizes.txt"
sample.name = "My paired reads"
distance = read.delim(sizeFile, h=F)[,1]
pdf(paste(sample.name, "insert.size.histogram.pdf", sep="."), w=11, h=8.5)
hist(distance, breaks=200, col="wheat", main=paste("Insert sizes for", sample.name), xlab="length (nt)")
dev.off()
Method 2: Calculate insert sizes with CollectInsertSizeMetrics function from picard (http://picard.sourceforge.net). This is also a good approximation for RNA samples.
# # I=File Input SAM or BAM file. (Required) # O=File File to write the output to. (Required) # H=File File to write insert size histogram chart to. (Required) # output: CollectInsertSizeMetrics.txt: values for -r and --mate-std-dev can be found in this text file # CollectInsertSizeMetrics_hist.pdf: insert size histogram (graphic representation) bsub java -jar /usr/local/share/picard-tools/CollectInsertSizeMetrics.jar I=foo.bam O=CollectInsertSizeMetrics.txt H=CollectInsertSizeMetrics_hist.pdf
[RNA-seq only] Get global coverage profile across transcripts
Do reads come from across the length of a typical transcript, or is there 3' or 5' bias (where most reads come from one end of a typical transcript)?
One way to look at this is with Picard's CollectRnaSeqMetrics tool
# Usage: java -jar /usr/local/share/picard-tools/CollectRnaSeqMetrics.jar INPUT=bamFile REF_FLAT=refFlatFile STRAND_SPECIFICITY=NONE OUTPUT=outputFile REFERENCE_SEQUENCE=/path/to/genome.fa CHART_OUTPUT=output.pdf VALIDATION_STRINGENCY=SILENT # Example command java -jar /usr/local/share/picard-tools/CollectRnaSeqMetrics.jar INPUT=WT.bam REF_FLAT=/nfs/genomes/mouse_mm10_dec_11_no_random/anno/refFlat.txt STRAND_SPECIFICITY=NONE OUTPUT=QC_metrics/WT.RnaSeqMetrics.txt REFERENCE_SEQUENCE=/nfs/genomes/mouse_mm10_dec_11_no_random/fasta_whole_genome/mm10.fa CHART_OUTPUT=QC_metrics/WT.RnaSeqMetrics.pdf VALIDATION_STRINGENCY=SILENT
The VALIDATION_STRINGENCY=SILENT option will keep the program from crashing if it finds something unexpected. The default: VALIDATION_STRINGENCY=STRICT
Interpreting quality control issues
See QC Fail Sequencing from the Babraham Institute
Attachments (3)
- cutadapt.jpeg (17.5 KB ) - added by 11 years ago.
- cutadapt.2.jpeg (109.1 KB ) - added by 11 years ago.
- fastq_phread-base.png (68.6 KB ) - added by 9 years ago.
Download all attachments as: .zip